Explain Different Types of Hydrogen Bond With Example
Medium Open in App Solution Verified by Toppr Hydrogen bond is a electrostatic attraction between a hydrogen atom which is bond to a more electronegative atom such as Nitrogen Oxygen fluorine. This type of bonding can be found in Hydrofluoric acid HF water H2O etc.
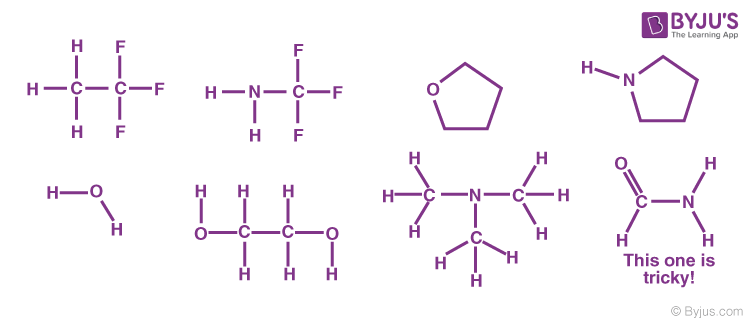
Hydrogen Bonding Properties Effects Types Examples Of Hydrogen Bond
Types of hydrogen bonds.

. Examples of such molecules are HF H2O NH3 in liqiud state C2H5OH ethanol and other alcohols and carboxylic acids like CH3COOH acetic acid. As a matter of fact any molecule that contains a hydrogen atom attached to an atom of nitrogen or oxygen is capable of such a bond. The hydrogen bond is weaker than a covalent bond and thus the bond length is longer.
Forming a bond doesnt neutralize the electrical nature of the participant atoms. When hydrogen bonding takes place between different molecules of the same or different. A hydrogen bond is an attractive force between the hydrogen atom of one molecule bound and more electronegative atoms of the same molecule or other molecules.
Lithium has one electron in its outer shell and fluorine has seven electrons in its outer shell. These are two types of hydrogen bonds - 1 Intermolecular Hydrogen bonding - It occurs between two separate molecules. Ethanol is one such alcohol that features hydrogen bonds.
Water is an ideal example of hydrogen bonding. Electrostatic force of attraction that exists between hydrogen atom of one molecule and electronegative atom of another molecule is called intermolecular hydrogen atom. Hydrogen bonding in HF In hydrogen fluoride HF the positive end of one dipole attracts the negative end of another similar dipole.
Bonds especially covalent bonds are often represented as. Similarly intramolecular hydrogen bonding occurs in selycylaldehyde and celycilic acid. Types of Bonds is an important topic with regard to Banking Awareness and the General Awareness part of the various Government exams conducted in the country.
Water ammonia and hydrogen fluoride are examples of such types of molecules. Compounds that have intramolecular hydrogen bonding usually have. In other words hydrogen bonding occurs only in compounds having the highly polar F-H O-H or N-H bond in their molecules.
Hydrogen bond formed between two different polar molecules of same or. Eg-Water is the most famous example - each water molecule can and does form 2 hydrogen bonds b Intra molecular hydrogen bond- This type of bond is formed between the hydrogen atom and the highly electronegative atom. Types of Hydrogen Bonding Intermolecular Hydrogen Bonding.
Ethanol and other alcohols contain hydrogen bonds. Polyurethane is an example of a polymer that contains hydrogen bonding between molecules. Nitrogen is a highly.
Fluorine is an. Hydrogen bonding in these molecules increases their tensile strength and melting point. Another example of an ionic bond is found in lithium fluoride LiF.
Electronegative atoms generally involved in. These are two types of. Examples include urea and polyurethane and the natural polymer cellulose.
Water methyl alcohol ethyl alcohol and sugar are examples of intermolecular hydrogen bonding. A water molecule is composed of a highly electronegative oxygen atom linked to the hydrogen atom. The hydrogen bonds in ammonia NH3 are formed between nitrogen and hydrogen atoms.
A Inter molecular hydrogen bond- This type of bond is formed between two different molecules of the same or different substances. This pictures shows examples of chemical bonding using Lewis dot notation. Eq - HFHFHF H bonding.
The hydrogen bonding which takes place within a molecule itself is called. The hydrogen bond is denoted by a dotted line. Hydrogen and carbon are not bonded while in water there is a single bond between each hydrogen and oxygen.
This results in a molecular dipole. Hydrogen Bonding Examples 1. Examples of Hydrogen Bonds Hydrogen bonds are found in nucleic acids between base pairs and between water molecules.
2 Intermolecular Hydrogen bonding - It occurs among the different facts of same molecule. O-nitrophenol and salicylic acid are examples of intramolecular hydrogen bonding. Intramolecular hydrogen bonding occurs in ortho nitrophenol.
Intramolecular Hydrogen Bonding. Candidates must know questions related to the financial terms are mostly asked in the Current Affairs General Awareness or the Banking Awareness section of all major Government exams especially Bank. In this situation an electron will be traded so that the outer shell of the lithium is full with eight electrons.
Explain different types of hydrogen bond giving examples. Explain different types of hydrogen bond giving examples. A hydrogen bond is a weak type of force that forms a special type of dipole-dipole attraction which occurs when a hydrogen atom bonded to a.
Diploe-Dipole Interaction In a covalent bond if there is an electronegativity difference between the two atoms then the electrons get pulled more towards the electronegative atom and it has a higher electron density. Sodium chloride or NaCl is an example of an ionic bond. 1 Intermolecular hydrogen bond.
1Intermolecular Hydrogen bonding When hydrogen bonding takes place between different molecules of the same or different. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. 683K views View upvotes Related Answer Gary Hiel.
Ethanol Alcohol Hydrogen bonds happen in alcohols. This is a. The hydrogen linking occurring between two or more similar or different molecules is called the intermolecular hydrogen bond.
The stability is comparatively low. Two with the hydrogen atoms and two with the.

What Are Types Of Hydrogen Bonds Explain With Suitable Examples Brainly In

Belum ada Komentar untuk "Explain Different Types of Hydrogen Bond With Example"
Posting Komentar